TESTS IN FOCUS
STORIES AT A GLANCE
Reticulocytes: Significance and Differentiation from RBC Inclusions
Dr Rimsha Imran, Hematology
Reticulocytes are immature red cells released from bone marrow that contain remnant cytoplasmic RNA and organelles like mitochondria and ribosomes. They mature in circulation over one-two days.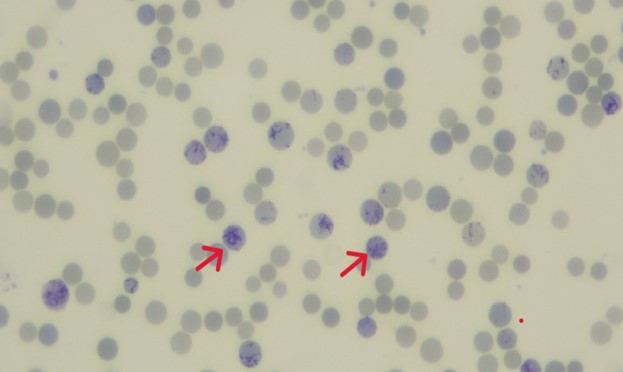
The reticulocyte count determines bone marrow erythropoietic activity in response to anemia. Reticulocytosis is seen in conditions like hemolytic anemias, hemorrhage, post-splenectomy and during recovery from anemia. They are visualized using supravital stains like methylene blue. On a blood film, reticulocytes (as highlighted by red arrows in the below figure) appear as pale blue containing dark blue reticular or granular material. It’s imperative to distinguish them from RBC inclusions like Howell-Jolly bodies, Heinz bodies, Pappenheimer bodies, and HbH inclusions during manual counting.
x------------------------x--------------------x


